 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
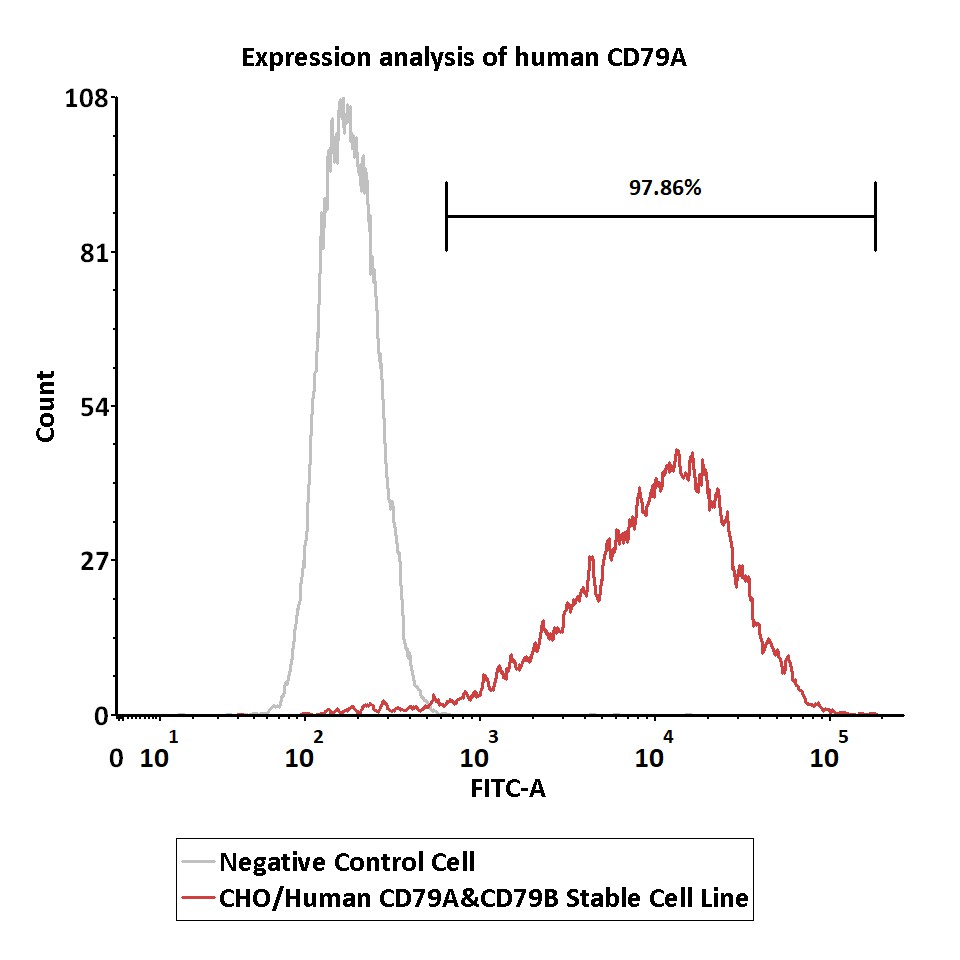
Expression analysis of human CD79A on CHO/Human CD79A&CD79B Stable Cell Line by FACS.
Intracellular staining was performed on CHO/Human CD79A&CD79B Stable Cell Line or negative control cell using FITC-labeled anti-human CD79A antibody after fixation and permeabilization.

Expression analysis of human CD79B on CHO/Human CD79A&CD79B Stable Cell Line by FACS.
Cell surface staining was performed on CHO/Human CD79A&CD79B Stable Cell Line or negative control cell using APC-labeled anti-human CD79B antibody.
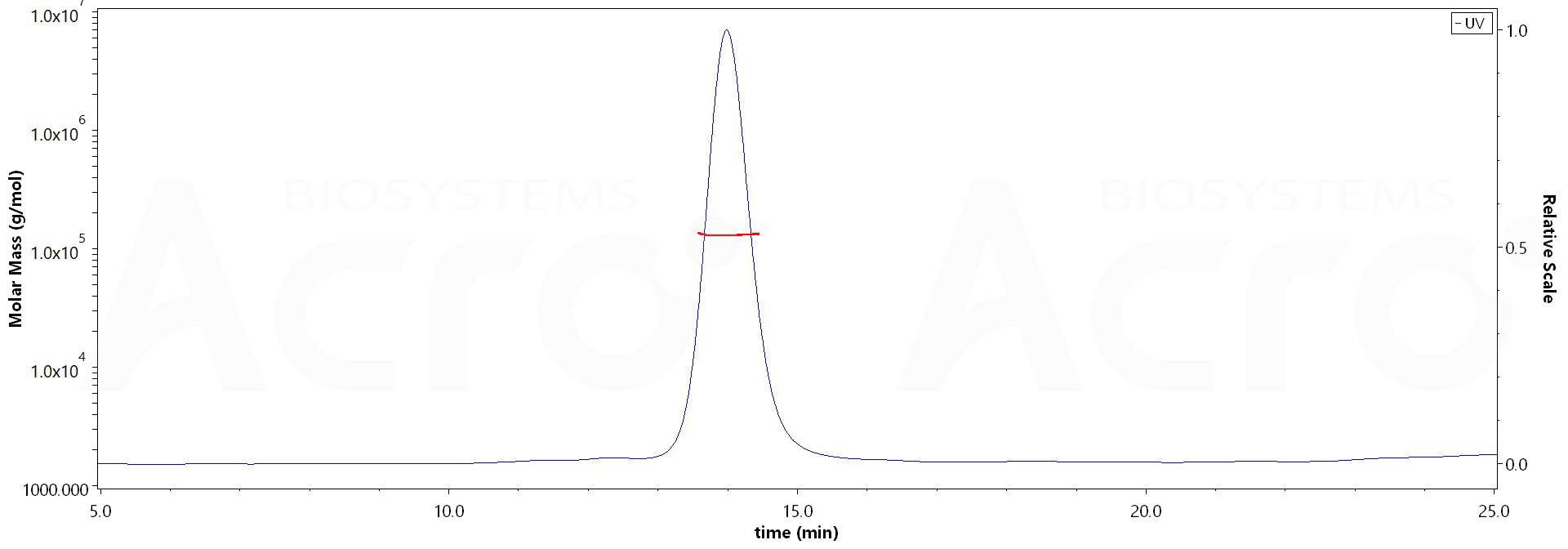
The purity of Human CD79A&CD79B Heterodimer Protein, Mouse IgG2a Fc,Flag Tag&Mouse IgG2a Fc,His Tag (Cat. No. CDB-H52W3) is more than 85% and the molecular weight of this protein is around 110-150 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Polatuzumab vedotin | RO-5541077-000; FCU-2711; DCDS-4501A; RG-7596; RO-5541077 | Approved | Genentech Inc | Polivy | United States | Lymphoma, Large B-Cell, Diffuse | Genentech Inc | 2019-06-10 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Polatuzumab vedotin | RO-5541077-000; FCU-2711; DCDS-4501A; RG-7596; RO-5541077 | Approved | Genentech Inc | Polivy | United States | Lymphoma, Large B-Cell, Diffuse | Genentech Inc | 2019-06-10 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| PRV-3279 | HDM-3002; MGD-010; PRV-3279; CD32BxCD79B | Phase 2 Clinical | Macrogenics Inc | Lupus Erythematosus, Systemic | Details |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| TolDCB29 | Phase 2 Clinical | Umc Utrecht | Arthritis, Rheumatoid | Details | |
| SHR-A1912 | SHR-A1912 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| CD79b-19 CAR T Cells Therapy (Massachusetts General Hospital) | Phase 1 Clinical | Massachusetts General Hospital | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details | |
| Autologous CD79b-targeting Chimeric Antigen Receptor T-cell Therapy(MD Anderson Cancer Center) | JV-213 | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Lymphoma | Details |
| NBT-508 | Phase 1 Clinical | Newbio Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD79b CAR-T cell therapy (Yake Biotechnology) | Phase 1 Clinical | Zhejiang University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Biphenotypic, Acute | Details | |
| Iladatuzumab vedotin | DCDS-0780A; RO-7032005 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Lymphoma, Non-Hodgkin | Details |
| PRV-3279 | HDM-3002; MGD-010; PRV-3279; CD32BxCD79B | Phase 2 Clinical | Macrogenics Inc | Lupus Erythematosus, Systemic | Details |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| TolDCB29 | Phase 2 Clinical | Umc Utrecht | Arthritis, Rheumatoid | Details | |
| SHR-A1912 | SHR-A1912 | Phase 2 Clinical | Shanghai Hengrui Pharmaceutical Co Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| CD79b-19 CAR T Cells Therapy (Massachusetts General Hospital) | Phase 1 Clinical | Massachusetts General Hospital | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details | |
| Autologous CD79b-targeting Chimeric Antigen Receptor T-cell Therapy(MD Anderson Cancer Center) | JV-213 | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, B-Cell; Lymphoma | Details |
| NBT-508 | Phase 1 Clinical | Newbio Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD79b CAR-T cell therapy (Yake Biotechnology) | Phase 1 Clinical | Zhejiang University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Biphenotypic, Acute | Details | |
| Iladatuzumab vedotin | DCDS-0780A; RO-7032005 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Lymphoma, Non-Hodgkin | Details |
This web search service is supported by Google Inc.